Catalytic Conversion of C1 Molecules (CO₂/CO, etc.)
Overview
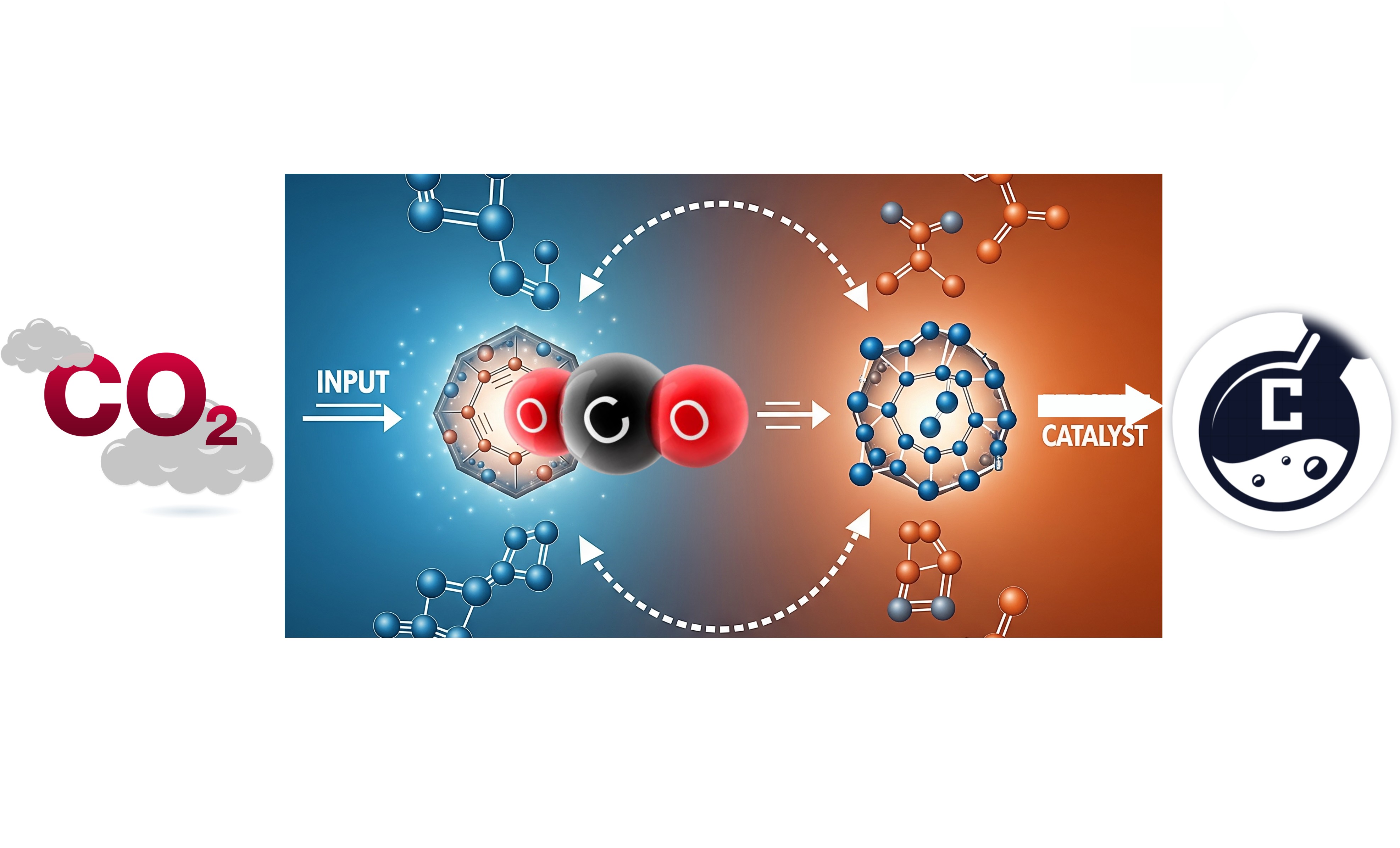
The urgent global demand to mitigate climate change and transition to a sustainable energy future has intensified interest in technologies capable of efficiently converting C₁ molecules—such as carbon dioxide (CO₂) and carbon monoxide (CO)—into high–energy-density carriers, including fuels and value-added chemicals. These molecules are abundant, inexpensive, and in the case of CO₂, a primary greenhouse gas. Transforming them into usable forms of energy not only provides an alternative to fossil fuels but also offers a pathway for carbon recycling, thereby reducing net greenhouse gas emissions. However, C₁ molecules are chemically stable and kinetically inert, requiring highly active and selective catalysts to drive their conversion under practical conditions. Developing catalytic materials with both high efficiency and long-term stability is therefore critical to achieving meaningful industrial application. Efficiency ensures that the process operates with minimal energy input and high product yields, while stability guarantees that catalysts maintain their performance over extended operation, reducing costs associated with frequent regeneration or replacement. Furthermore, the rational design of such catalysts cannot rely solely on empirical approaches; it demands in-depth mechanistic and kinetic studies to uncover the fundamental reaction pathways, rate-determining steps, and deactivation mechanisms. These insights allow researchers to tailor the active sites, electronic structures, and microenvironments of catalysts to optimize performance and selectivity. Understanding kinetics also enables precise control over reaction conditions, minimizing side reactions and improving process economics. Beyond the laboratory scale, these studies inform the scaling-up of processes by providing accurate models for reactor design and operation. In the broader context of low-carbon energy technologies, the integration of advanced catalytic systems into renewable-powered chemical manufacturing could enable the production of liquid fuels, synthetic natural gas, and other high-energy-density carriers from captured CO₂ or syngas derived from biomass, waste, or renewable electricity–driven electrolysis. Such carriers are essential for sectors where direct electrification is challenging, including aviation, shipping, and certain industrial processes. Moreover, the capability to store renewable energy in chemical form helps address intermittency issues associated with wind and solar power, improving grid stability and energy security. In summary, the strategic development of efficient, durable catalytic materials—guided by rigorous mechanistic and kinetic understanding—is indispensable for overcoming the inherent chemical inertness of C₁ molecules and for enabling their economically viable conversion into high-value products. This approach not only advances the frontier of catalysis research but also serves as a cornerstone technology for achieving global decarbonization goals, fostering a circular carbon economy, and ensuring a resilient, low-carbon energy infrastructure.